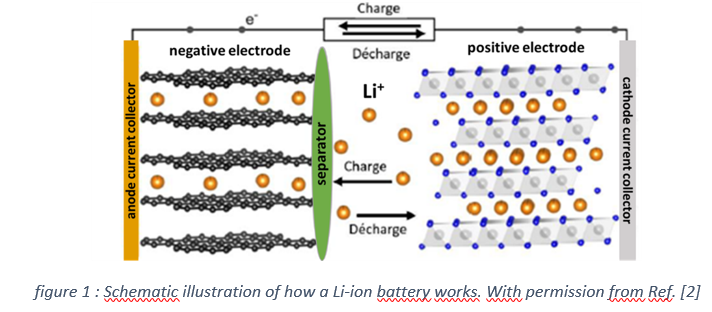
The world’s high demand for electrical energy calls for the development of advanced storage technologies. Li-ion batteries are currently the best performers in terms of specific energy and power density. The principle of this technology is to exchange lithium ions between a negative and a positive electrode by means of a separator impregnated of electrolyte that ensures electronic insulation between the electrodes but allows ionic diffusion of the lithium ions from one electrode to the other (figure 1).
Much attention has been paid to the chemical composition and atomic arrangement of the positive electrode to improve Li-Ion cell performances. Currently the active materials making up the positive electrode are mostly crystalline, and generally contain chemical elements that are critical from an environmental point of view, as well as in terms of abundance and cost (e.g. cobalt, lithium).
The work presented here is part of ESTELLA project aimed at developing positive electrodes for Li-ion batteries based on breakthrough materials such as glassy oxides [1], while guaranteeing energy densities exceeding 300 Wh/kg at the Li-Ion cell level.
The aim is to identify the most relevant physicochemical properties of glass that lead to improved electrochemical performance (energy density, cycling).
To better understand involved processes, the glasses studied are composed of three oxide families : a network-forming element (Si, B, P), a transition metal (Mn, Fe, …) and an alkali (Li, Na).
Our work shows that materials produced by the melt-quenching method are amorphous (XRD) and have specific microstructures (Raman Spectroscopy) depending on their chemical composition (SEM/EDX). Some parameters (eg. Tg, …) which can provide information on the mechanical behavior of the glasses’ atomic structure, was also determined (DTA). The comparison of these different glass properties with electrochemical characterizations of systems representative of Li-ion batteries will help guide our future development of positive electrode materials with the desired energy densities.
[1] A. K. Kercher et al., « Mixed polyanion glass cathodes: Glass-state conversion reactions », J. Electrochem. Soc., vol. 163, no 2, p. A131‑A137, 2016, doi: 10.1149/2.0381602jes.
[2] J. B. Goodenough et K.-S. Park, « The Li-Ion Rechargeable Battery: A Perspective », J. Am. Chem. Soc., vol. 135, no 4, p. 1167‑1176, janv. 2013, doi: 10.1021/ja3091438.