Radioactive waste vitrification has been carried out industrially in several countries for several decades. In these nuclear waste glasses, fission products and minor actinides are integrated in the vitreous network during high temperature reactions in vitrification furnaces. Meanwhile, research continues to investigates incorporation of new types of nuclear wastes which can lead to aggregates or crystals in glass matrix. During the steps of the process, the solid, liquid and gaseous states of the glass precursor and waste evolve continuously with time and temperature until a high-temperature homogeneous liquid is formed. After cooling, the waste remains integrated at the molecular scale in a homogeneous glass.
This study focuses on the fundamental research conducted to better describe the thermodynamics of these complex systems. Its objective is to better anticipate the behaviour of typical insoluble phases in prototypic nuclear glasses such as platinoid precipitates and complex molybdates that can form beyond their solubility limits.
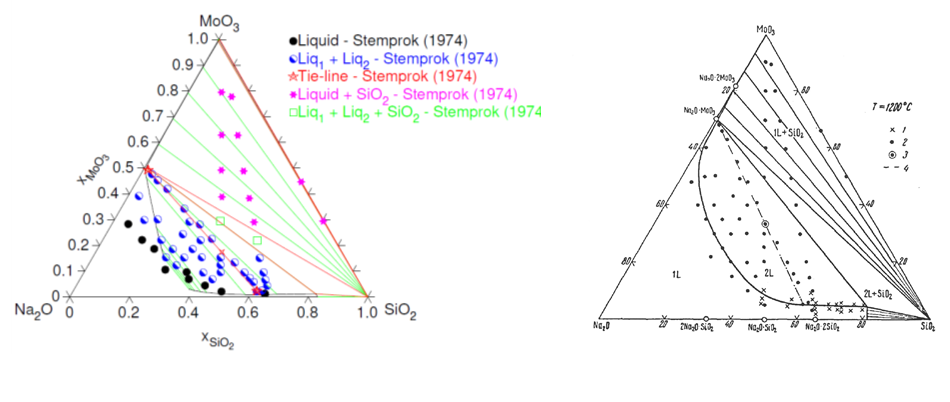
To predict the chemical interactions and the optimal conditions of these processes, a specific database, including the description of a simplified B2O3-Na2O-SiO2 glass melt, has been implemented using the Calphad method. This calculation tool allows to describe the phase equilibria and the phase separation phenomena between either platinoids or molybdates (Fig. 1) – mainly CaMoO4 and Na2MoO4 – and the glass melt. In parallel to a direct comparison between thermodynamic calculations and experimental results, this database is also used for a phase field approach. The objective of this coupling is to provide a consistent formalism between the thermodynamic properties of the chemical system and the local dynamics of the interface between the separate liquid-liquid phases of a heterogeneous glass.
[1] M. Stemprok et V. Jan, Homogenni Skla V Systemech,” Silikaty 1, 19 (1974).